
Effect of pH on microbiome
The attention paid to gut health in journals and scientific research has grown enormously. In fact, gut health is a necessity for achieving a significant reduction in antibiotic use. The role of gut bacteria goes beyond the fermentation of nutrients. More and more research is unravelling the complex interaction between bacteria and the immune system of the host in which they live.
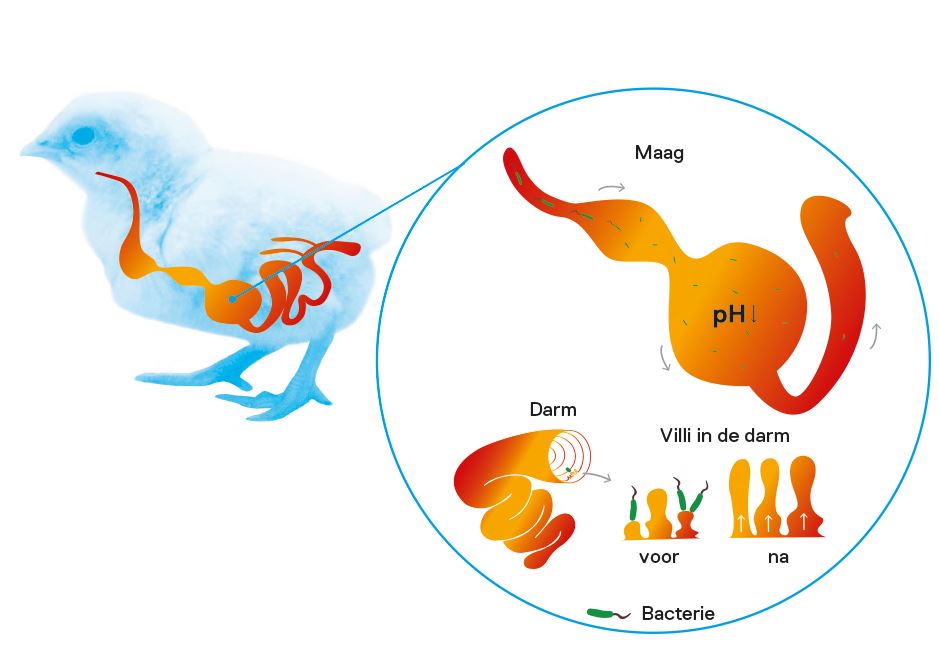
Which microbial organisms live in the gut, the so-called microbiome, is influenced by several factors. One important factor is the pH of the contents of the intestinal tract. Bacteria differ in their tolerance for a low pH. Salmonella, for example, is less acid tolerant compared to Lactobacillus species. In other words, if organic acid mixtures affect the pH, they also affect the composition of the microbiome.
Strong and weak acids
Organic acids differ in their acidity. This has a direct effect on their behaviour in the gastrointestinal tract. Strong acids ‘gladly’ give away their H+ molecule, thereby acidifying their surroundings. This happens partly in the water to which they are added and partly in the stomach. Weak acids, on the other hand, retain part of their H+ molecules and can therefore still actively acidify in the first part of the small intestine. A compound organic acid aims to have an effect in the water, the stomach and the intestine.
Buffered and unbuffered acids
There is also a difference between buffered organic acids and unbuffered organic acids. The unbuffered organic acids tend to dissociate more strongly than buffered acids. The final acidifying power is the same, but buffered acids will partly only inactivate (= dissociate) after the stomach and thus influence the pH in the first part of the intestine.
Taste
Every organic acid has its own taste. This is possibly an explanation for the fact that although most acid manufacturers give a recommended dosage of a pH=3.5 to 4, other manufacturers advise not to let the pH drop below 5. Although palatability is obviously very important, the risk of bacterial contamination of water with organic acids is significantly less when the pH is lower than 4.
Kanters and organic acids
The above-mentioned differences between organic acids is incomplete. It would go too far to explain all the differences here. During the last 35 years Kanters has specialised in the use of organic acids to support animal health and production. For each specific situation (water quality, animal species, age, husbandry system, etc) a specific acid mixture is most suitable.
For more information, please call 0499-425 600 or send an e-mail to mailto:info@kanters.nl




